Starting from 17 October 2023, the EU will enforce a new regulation (COMMISSION REGULATION (EU) 2023/2055) to restrict the use of intentionally added microplastics. The term ‘microplastic’ is not consistently defined, but is typically considered to refer to small, usually microscopic, solid particles made of a synthetic polymer. They are associated with long-term persistence in the environment, if released, as they are very resistant to (bio)degradation.
The Regulation establishes that substances on their own or, where the synthetic polymer microparticles are present to confer a sought-after characteristic, in mixtures in a concentration equal to or greater than 0,01 % by weight shall not be placed on the market.
The “intentionally added” microplastics have diverse technical functions and are used in various consumer, professional, and industrial products, including in cosmetic products and medical devices.
This regulation shall not apply to the placing on the market of some products, including medicinal and veterinary products, in vitro diagnostic devices; and shall not apply to the placing on the market of the following synthetic polymer microparticles, as substances on their own or in mixtures (point 5):
- synthetic polymer microparticles which are contained by technical means so that releases to the environment are prevented when used in accordance with the instructions for use during the intended end use;
- synthetic polymer microparticles the physical properties of which are permanently modified during intended end use in such a way that the polymer no longer falls within the scope of this entry;
- synthetic polymer microparticles which are permanently incorporated into a solid matrix during intended end use.
Reported below there is a timeline for the application of this regulation for cosmetic products and medical devices:
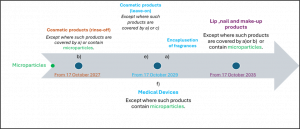
It is important underline that:
- Lip, nail and make-up products = From 17 October 2031 until 16 October 2035 suppliers of these products, containing synthetic polymer microparticles, shall provide the following statement: “This product contains microplastics.” However, products placed on the market before 17 October 2031 are not required to bear that statement until 17 December 2031.
- From 2027, suppliers of products containing synthetic polymer microparticles (medicinal, veterinary medicinal products; food additives and in vitro diagnostic devices), placed on the market for the first time to professional users and the general public, shall submit the following information to the Agency by 31 May of each year:
- a description of the end uses for which the synthetic polymer microparticles were placed on the market in the previous calendar year;
- for each end use for which the synthetic polymer microparticles were placed on the market, generic information on the identity of the polymers placed on the market in the previous calendar year;
- for each end use for which the synthetic polymer microparticles were placed on the market, an estimate of the quantity of synthetic polymer microparticles released to the environment in the previous calendar year, which shall include also the quantity of synthetic polymer microparticles released to the environment during transportation.
- for each use of synthetic polymer microparticles, a reference to the applicable derogation or derogations laid down in paragraph 4, (medicinal, veterinary medicinal products; food additives and in vitro diagnostic devices), or 5 point (previously presented in the text).
The following polymers are excluded from this restriction:
- polymers that are the result of a polymerisation process that has taken place in nature,
independently of the process through which they have been extracted, which are not chemically
modified substances;
- polymers that are degradable as proved in accordance with Appendix 15 of Regulation;
- polymers that have a solubility greater than 2 g/L as proved in accordance with Appendix 16 of Regulation;
- polymers that do not contain carbon atoms in their chemical structure.
References:
https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32023R2055#d1e726-67-1
https://echa.europa.eu/documents/10162/b56c6c7e-02fb-68a4-da69-0bcbd504212b







